Can I Take The Test At Home
Tests are available to detect chlamydia at home. Most at-home chlamydia tests are self-collection kits, which allow you to obtain a swab or sample of urine at home and return it to a laboratory by mail. If an at-home chlamydia test returns positive results, a doctor may suggest confirmation testing with a laboratory-based method.
Submission And Collection Notes
Do not use expired kits.
Prior to sampling, the patient should not have urinated for at least one hour. Female patients must not clean the labial area before collection. If the patient has used Replens® vaginal moisturizer, the specimen should be recollected as it will not be tested.
Collect approximately 10-50 mL of first-void urine in a sterile container. Using the provided disposable pipette, transfer urine from the sterile container to the Roche cobas® PCR Urine Sample Kit until the fluid level is between the two black fill lines. Tightly re-cap the Roche cobas®PCR Urine sample tube, and invert 5 times to mix. Urine specimens must be transferred into the Roche cobas® PCR Urine sample tube immediately for stabilization. If specimens cannot be transferred immediately, they can be stored at 2-30°C for up to 24 hours.
The Roche cobas®PCR Urine Sample Kits serve as a nucleic acid stabilizing transport and storage medium for urine specimens. The transferred urine must be between the black fill lines for accurate testing. Inadequate volumes will be cancelled.
If there is an excess of blood in the urine specimen , it should be discarded and recollected when appropriate. If received at the laboratory, these specimens will be cancelled. The Roche cobas®PCR Urine Sample Kits serve as a nucleic acid stabilizing transport and storage medium for urine specimens. The transferred urine must be between the black fill lines for accurate testing. Inadequate volumes will be cancelled.
Chlamydia And Gonorrhea Test
Quest Price: $105.88
Chlamydia is the most frequently reported bacterial sexually transmitted disease in the country. In 2002, over 800,000 cases of chlamydia were reported to the Center for Disease Control and Prevention .Gonorrhea is also a very common STD. The CDC estimates that more than 700,000 persons in the U.S. get new gonorrheal infections each year. The Chlamydia and Gonorrhea Test is a urine test that will detect the presence of bacteriums Chlamydia trachomatis and Neisseria gonorrhoeae.C. trachomatis infections are the leading cause of sexually transmitted diseases in the United States. C. trachomatis is known to cause cervicitis, Pelvic Inflammatory Disease , infant conjunctivitis, infant pneumonia, urethritis, epididymitis and proctitis. It is also the most frequent cause of non-gonococcal urethritis in men. Among women, the consequences of chlamydial infections are severe if left untreated. Approximately half of chlamydial infections are asymptomatic. Neisseria gonorrhoeae is the causative agent of gonorrhea. In men, this disease generally results in anterior urethritis accompanied by purulent exudate. In women, the disease is most often found in the cervix, but the vagina and uterus may also be infected.Positive results require further evaluation by your physician.Alternative Names: CT/GC, ProbeTec
You May Like: What Are The Early Signs Of Chlamydia
Dna Retrieval Characterization As Function Of Sample Ph
Performance of DNA capture system as a function of sample pH was characterized as follows. 1×PBS solution at a starting pH of 7.4 was titrated to prepare buffers at pH 3, 4, 5, 6, 7 and 8 using 0.1M NaOH and 1M HCl. For each pH, 50L of pH buffer was added to a mixture containing 180L lysis buffer, 20L magnetic particles and 20L lambda DNA stock . The mixture was divided into three 70L aliquots, and 30L binding buffer was added to each aliquot. The particles were subsequently washed twice with 100L wash buffer and eluted in 25L elution buffer for 5minutes. Each eluent was quantitated by absorbance measurement at 260nm using NanoDrop 2000. Retrieved DNA as a function of pH is presented in Fig. .
Droplet Magnetofluidic Lamp Assay Design
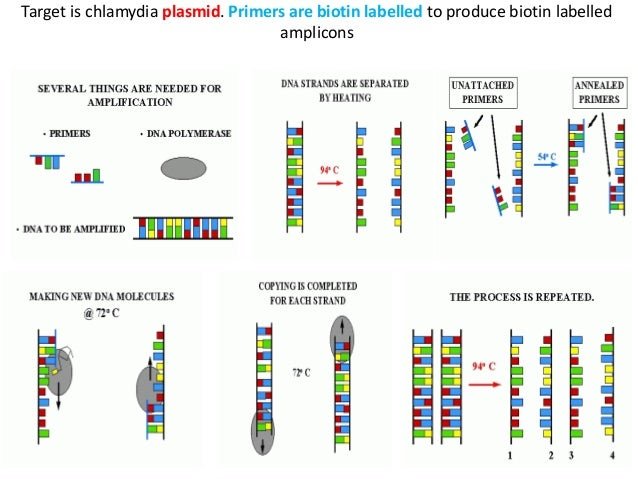
Valid target sequences for primer design were first identified using the NCBI GenBank database and were checked for cross-reactivity with other organisms prior to primer design. Using PrimerExplorer V4 , several primer sets were designed against various targets in the CT genome and were tested for threshold time and absolute signal difference from baseline . Based on the results, a primer set designed to target a 321-bp region located in the CT ompA gene was selected for subsequent experiments. All primers used for this experiment are presented in Table .
LAMP reaction was then characterized for temperature sensitivity and for the effect of sample preparation reagents on amplification . For each reaction, 10L synthetic target solution was mixed with input reagent composed of 50L lysis buffer, 10L resuspension buffer, 4L magnetic particles and 30L binding buffer. Each washing step used 25L wash buffer, followed by incubation in a 25L amplification mixture.
Also Check: Best Way To Get Rid Of Chlamydia
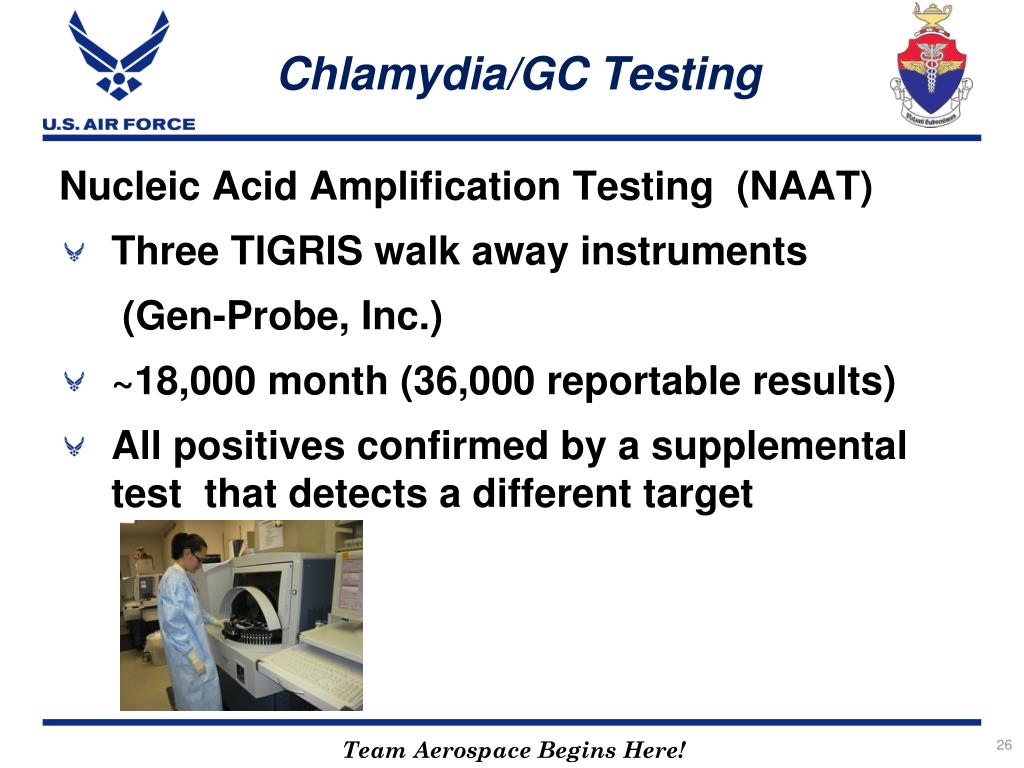
Nucleic Acid Amplification Test Chlamydia
Sticking to the principle of “Super Quality, Satisfactory service” ,We are striving to be a good business partner of you for Nucleic Acid Amplification Test Chlamydia, Test Swab Pcr, Rt Pcr Rapid Test, Omikron Test Reagent Kit,Pcr Test Naat. Our Company Core Principle: The prestige first The quality guarantee The customer are supreme. The product will supply to all over the world, such as Europe, America, Australia,Naples, Brazil,Turkey, Germany.We have a dedicated and aggressive sales team, and many branches, catering to our main customers. We are looking for long-term business partnerships, and ensure our suppliers that they will definitely benefit in both short and long run.
Nucleic Acid Amplification Tests For The Diagnosis Of Pharyngeal And Rectal Chlamydia And Gonorrhea Infections
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details. |
| First Posted : November 11, 2005Last Update Posted : September 27, 2012 |
- Study Details
| Device: Nucleic acid amplification test | Not Applicable |
In the latter part of the 1990’s, testing for Chlamydia trachomatis and Neisseria gonorrhoeae infections was revolutionized by the introduction of nucleic acid amplification tests which achieve greater sensitivity than traditional culture methods by exponentially replicating the nucleic acid of these organisms. The specimens collected for NAATs are easier to transport and NAATs permit collection of less invasive specimens for testing . Despite their somewhat higher costs, these tests have been found to be preferred by patients , by clinicians , and to permit expanded screening both in traditional clinical settings and at outreach sites where testing has not typically been done in the past.
Exclusion Criteria:
- History of receipt of antimicrobial agents active against N. gonorrhoeae or C. trachomatis in the preceding 30 days.
Recommended Reading: Can You Get Chlamydia Medicine Over The Counter
Clinical Sample Testing Conditions
Vaginal swab samples were collected from patients and archived in 80°C before use. The swab was expressed by gently dabbing the cotton tip against a 600L microcentrifuge tube containing ~200L lysis reagent composed of 50:20:1 lysis buffer, resuspension buffer and proteinase K solution by volume . Afterwards, the tube was inserted into an on-board thermal lysis chamber and incubated for 30minutes at 65°C followed by 15minutes at 95°C for proteinase inactivation. Swab samples tested at the emergency room were similarly expressed in lysis buffer without proteinase K and lysed using a portable microbead-beating unit . 70L of the lysate was transferred to the sample inlet of the droplet cartridge.
Questions For Your Doctor About Test Results
It can be helpful to bring questions to your doctor to learn more about your chlamydia test results. Helpful questions may include:
- What is my chlamydia test result?
- Did my test check for any other STDs?
- Do I need any treatment based on my results?
- How can I talk to my sexual partners about chlamydia?
- When should I be tested for STDs and how often?
Read Also: Chlamydia Clear Up On Its Own
How Much Does The Test Cost
The cost of chlamydia testing varies based on many factors. Chlamydia testing may be paid for by health insurance when ordered by a doctor. Because health plans vary, its important for patients to discuss the cost of testing, including any copays or deductibles, with their health plan.
For patients without health insurance coverage, the cost of testing may include the cost of the office visit and sample collection as well as technician fees. Testing may also be available for free or at low cost through community-based organizations and local health departments.
What To Think About
- If a chlamydia infection is suspected, do not have sexual intercourse until the test results have come back. If you have a chlamydia infection, do not have sexual intercourse for 7 days after the start of treatment. Your sex partner should also be treated for a chlamydia infection so that you don’t get reinfected and so that others don’t get infected.
- Only one laboratory test is needed to diagnose chlamydia. Your doctor can choose which test to use.
- Screening for and treating chlamydia can help prevent pelvic inflammatory disease . To learn more about the treatment of a chlamydia infection, see the topic Chlamydia.
- Other sexually transmitted infections may be present at the same time as chlamydia. So it is important to be tested and treated for all STIs. Chlamydia as well as other STIs can also increase the chance of getting human immunodeficiency virus . An HIV test may be offered at the same time as a test for chlamydia or other STIs.
You May Like: How To Cure Chlamydia Without Going To The Doctor
What Is This Test
This test detects bacterial DNA gene sequences in certain body fluids. This test is used when an infection with a bacteria called Chlamydia trachomatis is suspected in the urinary and genital organs. A sample of endocervical cells, urethral cells or discharge, or clean urine may be collected for this test.
Approach : Use Of A Different Naat For Confirmation Study 1
From January 2003 to September 2004, a moderate-prevalence population was tested for C. trachomatis. Swabs and FCU were collected from men and women seen in obstetrics/gynecology clinics, family planning clinics, or a sexually transmitted disease clinic in the San Francisco Bay area. Specimens were collected in BD’s ProbeTec transport tubes and originally tested by SDA. C. trachomatis-positive specimens were repeat tested by SDA, and then aliquots of the original specimens were tested by PCR and AC2. For PCR confirmation, 500 μl of the SDA sample was spun at 12,000 Ãg for 15 min, the supernatant was discarded, and the protocol for PCR swab processing and testing was followed. For AC2 confirmation, 100 μl of the SDA sample was inoculated into an AC2 swab transport tube and tested according to the package insert instructions.
Read Also: What Antibiotics Will Treat Chlamydia
Excellent Agreements Between The Sat Assay And The Qpcr Assay
After 19 of the 33 CT-positive, 19 of the 32 NG-positive, 19 of the 29 UU-positive, and 29 pathogen-negative samples were tested by SAT, 100% agreements between the SAT assay and the qPCR assay were observed in samples with a concentration more than 1×103 copies/ml, as shown in Table . Specifically, the sensitivity and specificity were 100% and 100% for CT, 100% and 100% for NG, and 100% and 100% for UU, respectively. No discrepancy was found. In addition, SAT exhibited a good reproducibility. Specifically, the coefficient of variations of CT, NG, and UU were 1.91, 2.94, and 1.25% for high concentration of positive controls, and 3.14, 0.28, and 2.28% for low concentration of positive controls, respectively.
Table 1 Comparison of CT, NG, and UU results in samples with concentration more than 1×103 copies/ml
What Does The Test Measure
Chlamydia testing looks for evidence of infection with the bacteria Chlamydia trachomatis. There are several types of tests that can be used to detect chlamydia, including molecular testing, also called Nucleic Acid Amplification Test , and cell culture.
NAAT is the preferred method for detecting a chlamydia infection. This type of test detects the genetic material of Chlamydia trachomatis. It can be performed using a urine sample or swab of fluid taken from a site of potential infection such as the urethra, vagina, rectum, or eye.
Traditionally, NAAT takes a day or more to provide results, but there have also been rapid chlamydia tests developed using NAAT methods. Rapid chlamydia tests can often provide a result within 30 to 90 minutes. Rapid chlamydia tests are typically performed on urine samples or swabs of fluid taken from the vagina or cervix.
Although much less commonly used, cell cultures can help diagnose a chlamydia infection. Chlamydia cell cultures may be used in children with a suspected chlamydia infection, when evaluating potential infections in the anus or rectum, and when initial treatment for chlamydia is unsuccessful. In these cases of treatment failure, doctors may use a cell culture to help understand which treatments may be most effective for an individuals infection.
Other types of chlamydia tests are available but are rarely used given the accuracy and availability of NAAT.
You May Like: How Do You Get Tested For Chlamydia
Why It Is Done
A test for chlamydia is done to:
- See whether symptoms of a sexually transmitted infection are caused by a chlamydia infection.
- Check people who are at high risk for being infected with chlamydia. A chlamydia infection does not always cause symptoms. The Public Health Agency of Canada recommends checking for chlamydia for:
- All sexually active women age 25 or younger.
- Women and men with high-risk sexual behaviours.
- All pregnant women in the first trimester and again in the third trimester if high-risk sexual behaviours are reported. Treating a pregnant woman who has a chlamydia infection can prevent an infection in her newborn.
Patient And Public Involvement
Vaginal specimens are an acceptable and sometimes a preferred way for chlamydia and gonorrhoea testing among women. In this study, we compare the evidence base of performance of different testing sites for detection of chlamydia and gonorrhoea by conducting a systematic review of published primary research. We did not involve patient groups in the design or conduct of the study. The findings of the study can be used to standardise testing practices.
Also Check: Can You Find Chlamydia In A Blood Test
Are Test Results Accurate
Although chlamydia testing is an important method of finding and treating this common STD, test results could be impacted by the following:
- The use of antibiotics within several days before testing
- Urinating within one hour of sample collection
- Vaginal douching within 24 hours of testing
- Improper sample collection
- Contamination of rectal samples with fecal matter
Design And Fabrication Of Mobinaat Droplet Magnetofluidic Cartridge
The mobiNAAT cartridge was fabricated by assembling four layers of PMMA sheets as outlined in Fig. . The chamber layer was laminated with PTFE tape on one side in order to render the surface hydrophobic. The sheet was subsequently cut and laminated with the upper layer to generate hydrophilic PMMA chambers. The lower layer was laminated with PTFE film and engraved in order to expose the PMMA surface along the perimeter for bonding. A spacer frame was lined on both faces with acrylic pressure-sensitive adhesive film to provide a watertight seal and spacing between the upper and lower layers. The top layer was pre-assembled with chamber and spacer layers to form a single component, loaded with assay reagents and bonded with the lower layer. The cartridge was then flipped right side up and filled with approximately 100L FC-40 fluorinated oil. Afterwards, a mixture of 30L binding buffer and 4L magnetic particles was loaded into the sample chamber and sealed at the inlet port with plastic tape prior to transportation. Prior to each assay, sample lysate was loaded into the sample chamber of the cartridge using a disposable pipette.
Don’t Miss: How Quick Can You Catch Chlamydia
How Should I Get Ready For The Test
Endocervical cells:
Tell the healthcare worker if you have a medical condition or are using a medication or supplement that causes excessive bleeding. If possible, schedule the procedure one week after your menstrual period. Do not douche or have sexual intercourse for 24 hours before the procedure.
You may be asked to urinate prior to your endocervical sampling. This will make it easier for the healthcare worker to see your cervical canal during the procedure and may make the procedure more comfortable for you.
Urethral cells/discharge:
Before collection of urethral cells and/or urethral discharge for this test, you should not urinate during the hour prior to the test. This is because urine may minimize or clear your urethra of any organisms , thus affecting the results of this test.
Urinate an hour or more before a sample is collected.
Clean urine:
To prepare for giving a urine sample, be sure to drink enough fluids before the test, unless you have been given other instructions. Try not to empty your bladder before the test.
Urinate an hour or more before a sample is collected.
What Are Normal Results For This Test
Laboratory test results may vary depending on your age, gender, health history, the method used for the test, and many other factors. If your results are different from the results suggested below, this may not mean that you have a disease. Contact your healthcare worker if you have any questions. The following is considered to be a normal result for this test:
- Negative
Don’t Miss: Is Chlamydia And Gonorrhea The Same Thing